Abstract
BACKGROUND: The thrombopoietin receptor agonist (TPO-RA) eltrombopag was approved for the treatment of chronic immune thrombocytopenia (cITP), >12 months after initial ITP diagnosis. The registration trials however included patients with ITP duration >6 months. Moreover, with increasing familiarity and confidence with the use of TPO-RAs in ITP, the use of these agents in real world has not been restricted to cITP.
OBJECTIVE: The objective of this study was to describe treatment characteristics of eltrombopag use among adult patients diagnosed with ITP initiating treatment ≤6, 7-12, and >12 months after their initial ITP diagnosis in the real-word setting.
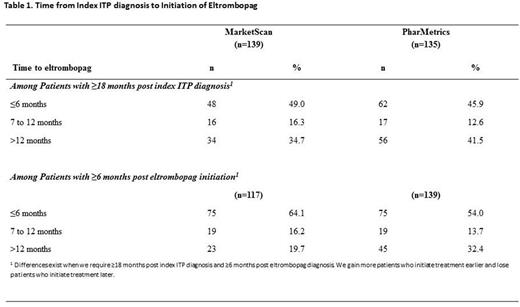
METHODS: A retrospective cohort study was conducted using MarketScan® - Commercial and Medicare Supplemental Database, and PharmetricsPlusTM Adjudicated Claims Database. Adult patients (≥18 years) with ≥2 non-diagnostic outpatient claims separated by ≥30 days or ≥1 inpatient claim carrying a diagnosis code for ITP during Jul 1, 2010 - Jun 30, 2014 were eligible for inclusion. The index date was the date of the first ITP diagnosis. Patients were excluded if they had continuous enrollment in medical and pharmacy benefits for <24 months prior and <18 months following the index date, or had a medical claim for secondary thrombocytopenia during the pre-index period. Timing of eltrombopag initiation was calculated from the index date to the date of first eltrombopag claim. Patients were categorized into 1 of 3 cohorts, those initiating treatment ≤6, 7-12, or >12 months after index date. Treatment outcomes were assessed among a subset of patients regardless of follow-up period post the index ITP diagnosis but who had ≥6 months of continuous enrollment in medical and pharmacy benefits after initiating treatment with eltrombopag; treatment duration, medication possession ratio (MPR), time to treatment discontinuation and time to treatment restart were compared among patients initiating treatment ≤6, and 7-12 after ITP diagnosis to those initiating treatment >12 months after their index ITP diagnosis. MPR was calculated as the sum of the days' supply for all fills divided by the number of days between the first and last fill plus days' supply of the last fill. Outcomes compared between cohorts using t-tests and chi-square tests. RESULTS: A total of 7,068 patients - 3,264 patients in MarketScan and 3,804 in PharMetrics - were eligible for inclusion in the study; 117 (3.6%) and 139 (3.7%), respectively, received eltrombopag. More than half of patients in both MarketScan and PharMetrics initiated treatment ≤12 months after their index diagnosis (see Table 1). The average treatment durations were similar among patients initiating treatment ≤6 months (120.8±57.1; P =0.456) and 7-12 (110.5±59.5; P =0.991) months after index diagnosis compared to those initiating treatment >12 months after index diagnosis (110.3±64.7). The MPR was 0.9 in all 3 cohorts; 84.0%, 73.7% and 78.3% patients initiating treatment ≤6 months, 7-12 months and > 12months after index diagnosis, respectively were considered to be adherent based upon an MPR≥0.8. The proportion of patients who discontinued treatment were not statistically significant between cohorts (≤6 months: 53.3% [ P =0.816]; 7-12 months: 57.9% [ P =1.000]; >12 months 56.5%). Time to treatment discontinuation was statistically longer among patients initiating treatment ≤6 months compared to those initiating treatment >12 months after index diagnosis (63.7±37.3 days vs 39.5±26.4 days; P =0.035); however the difference between patients initiating treatment 7-12 and >12 months after index diagnosis (50.3±30.5 days vs 39.5±26.4 days; P =0.365) was not significant. Twelve percent ( P =0.307), 26.3% ( P =1.000) and 21.7% of patients initiating treatment ≤6 months, 7-12 and >12 months after index diagnosis restarted eltrombopag. The average time to treatment restart was approximately 50 days across all 3 cohorts. CONCLUSION: Although eltrombopag is currently approved for patients with chronic ITP, results of this study indicate that in real world setting physicians have been using it in patients with newly diagnosed and persistent ITP, with overall treatment patterns being very similar to those in patients with cITP. These data, supported by other real world studies underscore the clinical utility of eltrombopag in early-stage ITP and warrant further exploration in more robust prospective studies.
Stafkey-Mailey: Xcenda: Employment. Roy: Novartis: Employment, Equity Ownership. Lokhandwala: Xcenda: Employment. Yuan: Xcenda: Employment. Landsman-Blumberg: Xcenda: Employment. Siddiqui: Novartis: Employment, Equity Ownership. Ghaznawi: Novartis: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal